
the world’s largest RCO
We revolutionized the traditional model, creating an entirely new system that redefines what’s possible for you and your organization
RCO Vs CRO: Drug and Device Development Outsourcing 2.0
Traditional CRO push a full-service, "cookie-cutter" agenda, offering little compromise for the small Sponsor; ProPharma’s Research Consulting Organization RCO model leads with strategy to help de-risk programs and create tailored solutions to maximize probability of success.
ProPharma has transformed its organizational structure and solutions to put its clients at the very center. We offer a suite of bespoke consulting solutions across service lines and functional areas of expertise to span the full product life cycle. ProPharma embraces partnerships to reduce delays and drive consistency with dedicated and experienced strategists and program managers for end-to-end oversight.
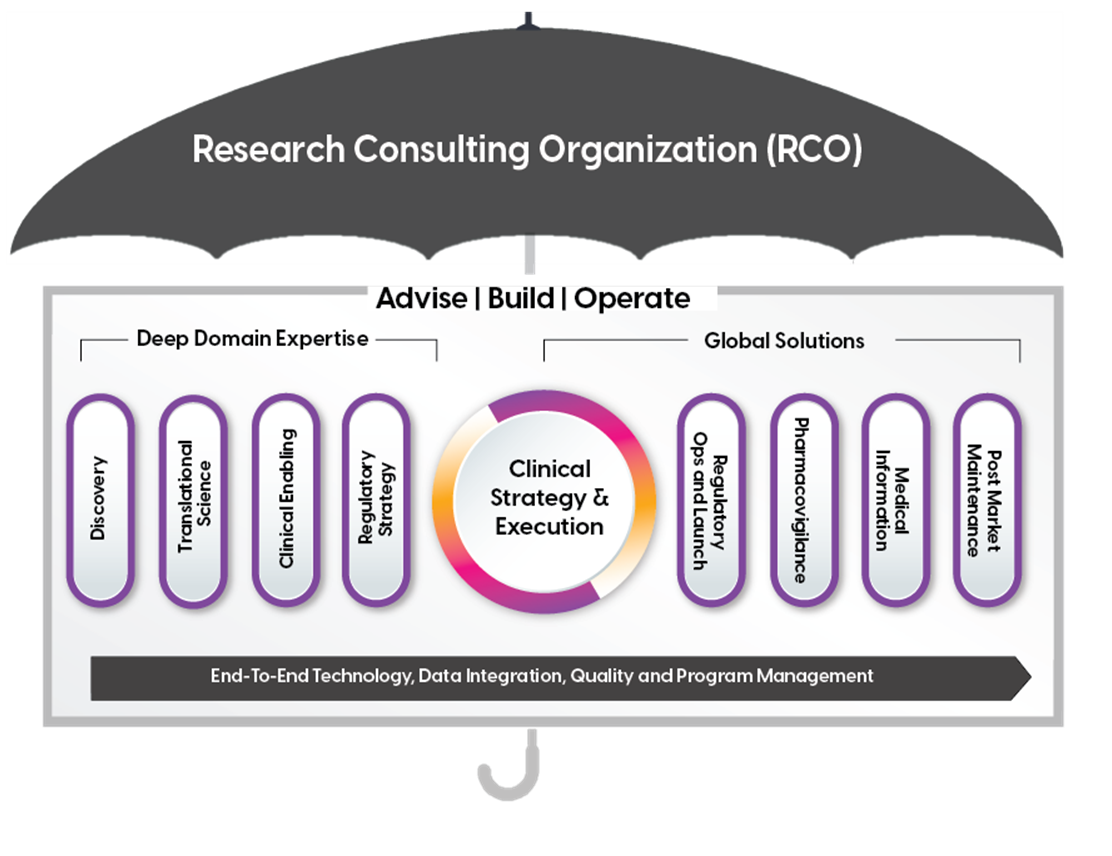
Our Approach
As the industry pioneer, ProPharma partners with its clients by providing targeted subject matter experts, world-class strategists, and dedicated program managers to co-create optimal solutions. Fueled by deep domain expertise, we provide end-to-end support across the full product lifecycle.
Better Solutions.
Innovative Partners.
Custom Solutions for
Complex Needs.
Innovation is
Our Foundation.
Bespoke Strategy.
Exceptional Service.

About Us
As the world’s largest RCO, ProPharma helps companies excel at every stage of the pharmaceutical lifecycle. We collaborate with clients to co-create optimal solutions, build sustainable and scalable operating models, develop auxiliary capabilities, and position our clients for development and growth...and we do it with speed & consistency, at scale. A true partner from start to finish, we help clients solve complex challenges in a dynamic environment
Leading Transformation Since 2001
ProPharma has been growing companies since 2001 through partnership, leveraging new acquisitions, and building creative solutions at the intersection of talent and capabilities.
2001
2007
2011
2014
2015
2017
2018
2019
2021
2022
2023
Founded
ProPharma Group
Established as a leading provider of validation and other related life science consulting services in the pharmaceutical, biotechnology, and medical device industries.
Merged
Computer Compliance
Joined forces with Computer Compliance to deliver an enhanced experience in validation of automated systems, computers, and IT services.
Acquired
Advanced Response Management (ARM)
Expanded our suite of services to include medical information with the acquisition of Advanced Response Management (ARM), based in the United States.
Acquired
Professional Information Ltd
Further expanded our medical information capabilities with the acquisition of Professional Information Ltd (PI) operating in the United Kingdom and Australia.
Acquired
PROSAR
Acquired the leading provider of pharmacovigilance services, PROSAR, based in the United States, to complement our suite of MI services.
Acquired
Drug Safety Solutions
Further expanded our pharmacovigilance services with the acquisition of Drug Safety Solutions, a clinical safety company, based in the United States.
Expanded
Global Support
Expanded global support with new contact center in Tokyo, Japan.
Acquired
SOLUTIONS in Health
Acquired SOLUTIONS in Health, a Canada-based provider of medical information services.
Acquired
European-Based Xendo Life Sciences
Expanded our global reach and service offerings for compliance consulting, regulatory sciences, and pharmacovigilance with the acquisition of European-Based Xendo, which included Sofus Regulatory Affairs.
Acquired
The Weinberg Group and Southwood Research
Expanded our support for clients throughout all phases of the product lifecycle with the acquisitions of The Weinberg Group, a leading provider of regulatory sciences services based in the United States and Southwood Research, a pre-approval regulatory science services provider based in the United Kingdom.
Expanded
Global ProPharma Brand
United Xendo, Sofus Regulatory Affairs, and SOLUTIONS in Health under the global ProPharma brand.
Acquired
Pharmica Consulting and Intrinsic Clinical Systems
Acquired Pharmica Consulting, a life science consulting company that provides Project Management (PM) consulting solutions and proprietary operations software to pharmaceutical and biotech companies for the execution of clinical trials.
Acquired
iSafety Systems
Acquired iSafety Systems, a leading pharmacovigilance provider headquartered in India’s leading biotech hub, Hyderabad.
Merger
The Planet Group
ProPharma and The Planet Group, a leading provider of specialized consulting services and outsourced human capital solutions to the Life Sciences, Energy/Engineering, and Technology sectors, announced an agreement to merge the two companies.
Acquired
Diamond Pharma Services
Acquired Diamond Pharma Services, a leading regulatory sciences, pharmacovigilance, and compliance and quality consultancy headquartered in the United Kingdom, with offices in Europe.
Acquired
M Squared Associates (M2)
M Squared Associates (M2), an industry leading clinical, regulatory, and quality consulting firm serving the medical device and diagnostic industry.
Acquired
OneSource Regulatory (OSR) and OneSource Regulatory Technology (OSR-T)
OSR is an industry leading source for regulatory, medical, marketing operations, and compliance consulting services, including promotional and labeling review, and technology solutions.
Acquired
Kateric
Since 2004, Kateric has established a reputation as a strategic partner throughout the life sciences industry providing worldwide access to regulatory documentation services and capabilities over a range of product lifecycle needs
Acquired
Digital Lab Consulting
Since 2018, Digital Lab Consulting (DLC) has been a strategic partner, providing deep industry and digital transformation expertise to enable organizations to transform their business through better use of data and technology. DLC’s expertise covers a wide range of skill sets and capabilities across the pharma value chain.
Acquired
Clinres Farmacija
Clinres Farmacija is a leading Contract Research Organization based in Croatia. This move reinforces ProPharma's commitment to expanding its presence in Central and Eastern Europe, providing comprehensive clinical services.
2001
Founded
ProPharma Group
Established as a leading provider of validation and other related life science consulting services in the pharmaceutical, biotechnology, and medical device industries.
2007
Merged
Computer Compliance
Joined forces with Computer Compliance to deliver an enhanced experience in validation of automated systems, computers, and IT services.
2011
Acquired
Advanced Response Management (ARM)
Expanded our suite of services to include medical information with the acquisition of Advanced Response Management (ARM), based in the United States.
2014
Acquired
Professional Information Ltd
Further expanded our medical information capabilities with the acquisition of Professional Information Ltd (PI) operating in the United Kingdom and Australia.
2015
Acquired
PROSAR
Acquired the leading provider of pharmacovigilance services, PROSAR, based in the United States, to complement our suite of MI services.
2017
Acquired
Drug Safety Solutions
Further expanded our pharmacovigilance services with the acquisition of Drug Safety Solutions, a clinical safety company, based in the United States.
Expanded
Global Support
Expanded global support with new contact center in Tokyo, Japan.
2018
Acquired
SOLUTIONS in Health
Acquired SOLUTIONS in Health, a Canada-based provider of medical information services.
Acquired
European-Based Xendo Life Sciences
Expanded our global reach and service offerings for compliance consulting, regulatory sciences, and pharmacovigilance with the acquisition of European-Based Xendo, which included Sofus Regulatory Affairs.
2019
Acquired
The Weinberg Group and Southwood Research
Expanded our support for clients throughout all phases of the product lifecycle with the acquisitions of The Weinberg Group, a leading provider of regulatory sciences services based in the United States and Southwood Research, a pre-approval regulatory science services provider based in the United Kingdom.
Expanded
Global ProPharma Brand
United Xendo, Sofus Regulatory Affairs, and SOLUTIONS in Health under the global ProPharma brand.
2021
Acquired
Pharmica Consulting and Intrinsic Clinical Systems
Acquired Pharmica Consulting, a life science consulting company that provides Project Management (PM) consulting solutions and proprietary operations software to pharmaceutical and biotech companies for the execution of clinical trials.
Acquired
iSafety Systems
Acquired iSafety Systems, a leading pharmacovigilance provider headquartered in India’s leading biotech hub, Hyderabad.
Merger
The Planet Group
ProPharma and The Planet Group, a leading provider of specialized consulting services and outsourced human capital solutions to the Life Sciences, Energy/Engineering, and Technology sectors, announced an agreement to merge the two companies.
Acquired
Diamond Pharma Services
Acquired Diamond Pharma Services, a leading regulatory sciences, pharmacovigilance, and compliance and quality consultancy headquartered in the United Kingdom, with offices in Europe.
2022
Acquired
M Squared Associates (M2)
M Squared Associates (M2), an industry leading clinical, regulatory, and quality consulting firm serving the medical device and diagnostic industry.
Acquired
OneSource Regulatory (OSR) and OneSource Regulatory Technology (OSR-T)
OSR is an industry leading source for regulatory, medical, marketing operations, and compliance consulting services, including promotional and labeling review, and technology solutions.
Acquired
Kateric
Since 2004, Kateric has established a reputation as a strategic partner throughout the life sciences industry providing worldwide access to regulatory documentation services and capabilities over a range of product lifecycle needs
2023
Acquired
Digital Lab Consulting
Since 2018, Digital Lab Consulting (DLC) has been a strategic partner, providing deep industry and digital transformation expertise to enable organizations to transform their business through better use of data and technology. DLC’s expertise covers a wide range of skill sets and capabilities across the pharma value chain.
Acquired
Clinres Farmacija
Clinres Farmacija is a leading Contract Research Organization based in Croatia. This move reinforces ProPharma's commitment to expanding its presence in Central and Eastern Europe, providing comprehensive clinical services.
Our leaders forge new industry paths
with expertise and dedication
ProPharma is fortunate to have an exceptional leadership team with deep industry experience as well as passion
and drive around our higher purpose of improving patient health and safety.
“Working in close partnership with our clients to improve the health and safety of patients, our global teams are committed to quality and excellence in everything we do to support clients throughout the full product lifecycle.”
Michael Stomberg
Chief Executive Officer

“Working in close partnership with our clients to improve the health and safety of patients, our global teams are committed to quality and excellence in everything we do to support clients throughout the full product lifecycle.”
Michael Stomberg
Chief Executive Officer


Discover a driven and collaborative culture
We are a group of innovators, thought leaders, and curious minds from diverse backgrounds across the globe. Our success is measured how we serve our customers, support our communities, and drive quality outcomes for our clients and sponsors.
As the world’s largest RCO, we revolutionized the traditional model, creating an entirely new system that redefines what’s possible for you and your career.
Awards
Learn more about our accomplishments as industry leaders in innovation, strategic excellence, corporate stewardship, and intelligent work as recognized by organizations around the world.

ProPharma Announces the Appointment of Vicki Gashwiler as Vice President of Clinical Operations, Medical Technology
RALEIGH, January 24, 2024, ProPharma Group (ProPharma), the leading global provider of regulatory, clinical, and compliance services for the life sciences industry, and a portfolio company of Odyssey...

ProPharma Unveils Prodigy: Groundbreaking Technology-Enabled Consulting Platform to Revolutionize Life Science Consulting Industry
Whitepaper published alongside today's announcement details $5M+ investment and up to 50% improvement in speed, quality, and cost with new AI-enhanced solutions. RALEIGH, NC, January 4, 2023 –...
Awards
Learn more about our accomplishments as industry leaders in innovation, strategic excellence, corporate stewardship, and intelligent work as recognized by organizations around the world.

ProPharma Announces the Appointment of Vicki Gashwiler as Vice President of Clinical Operations, Medical Technology
RALEIGH, January 24, 2024, ProPharma Group (ProPharma), the leading global provider of regulatory, clinical, and compliance services for the life sciences industry, and a portfolio company of Odyssey...

ProPharma Unveils Prodigy: Groundbreaking Technology-Enabled Consulting Platform to Revolutionize Life Science Consulting Industry
Whitepaper published alongside today's announcement details $5M+ investment and up to 50% improvement in speed, quality, and cost with new AI-enhanced solutions. RALEIGH, NC, January 4, 2023 –...
Bespoke Strategy. Global Scale.
Strategically positioned in hubs across 5 continents, we’re changing up the standard approach to lifecycle development. Our global reach powers the insights that are key to your success.







